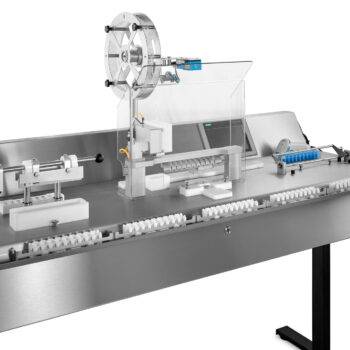
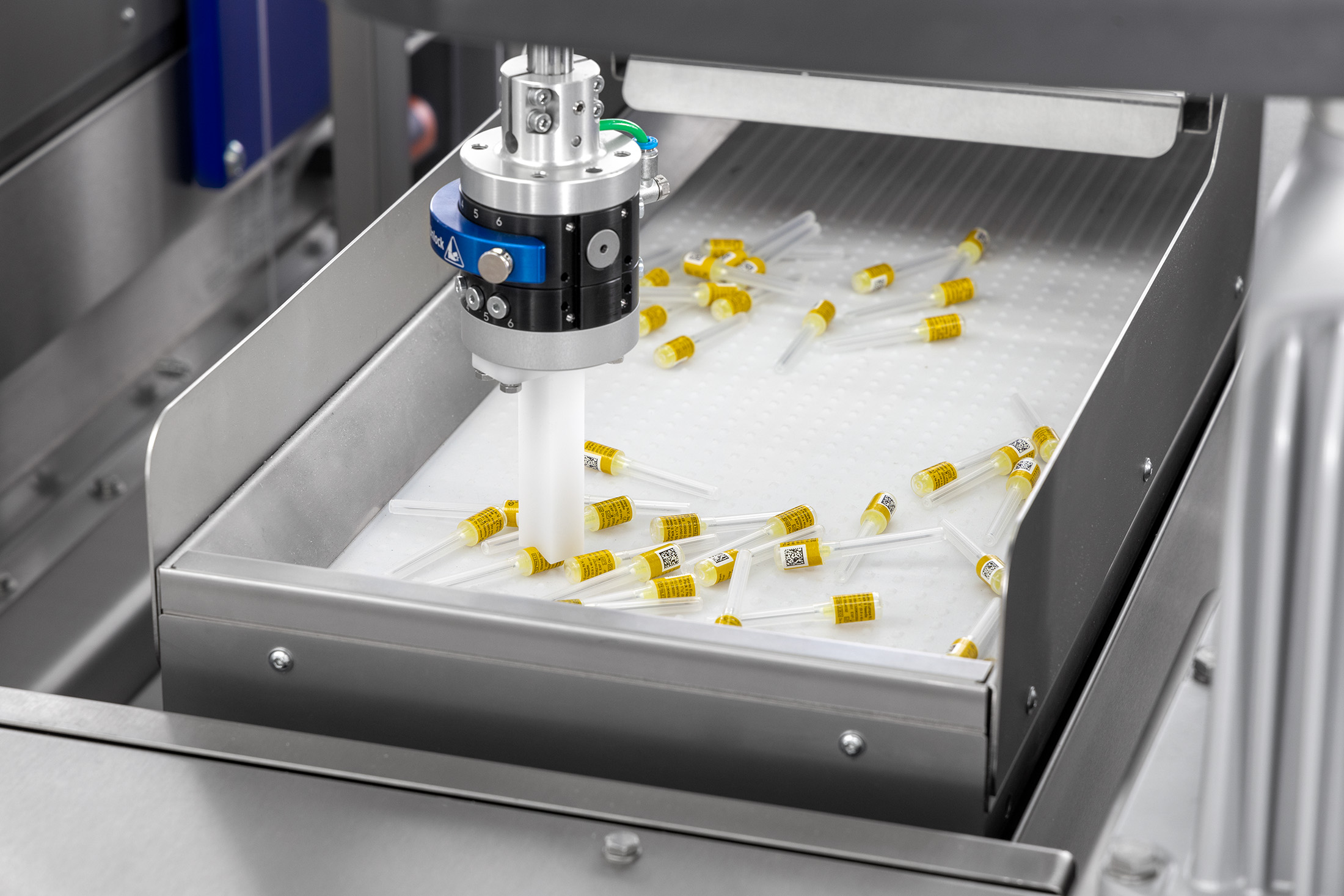
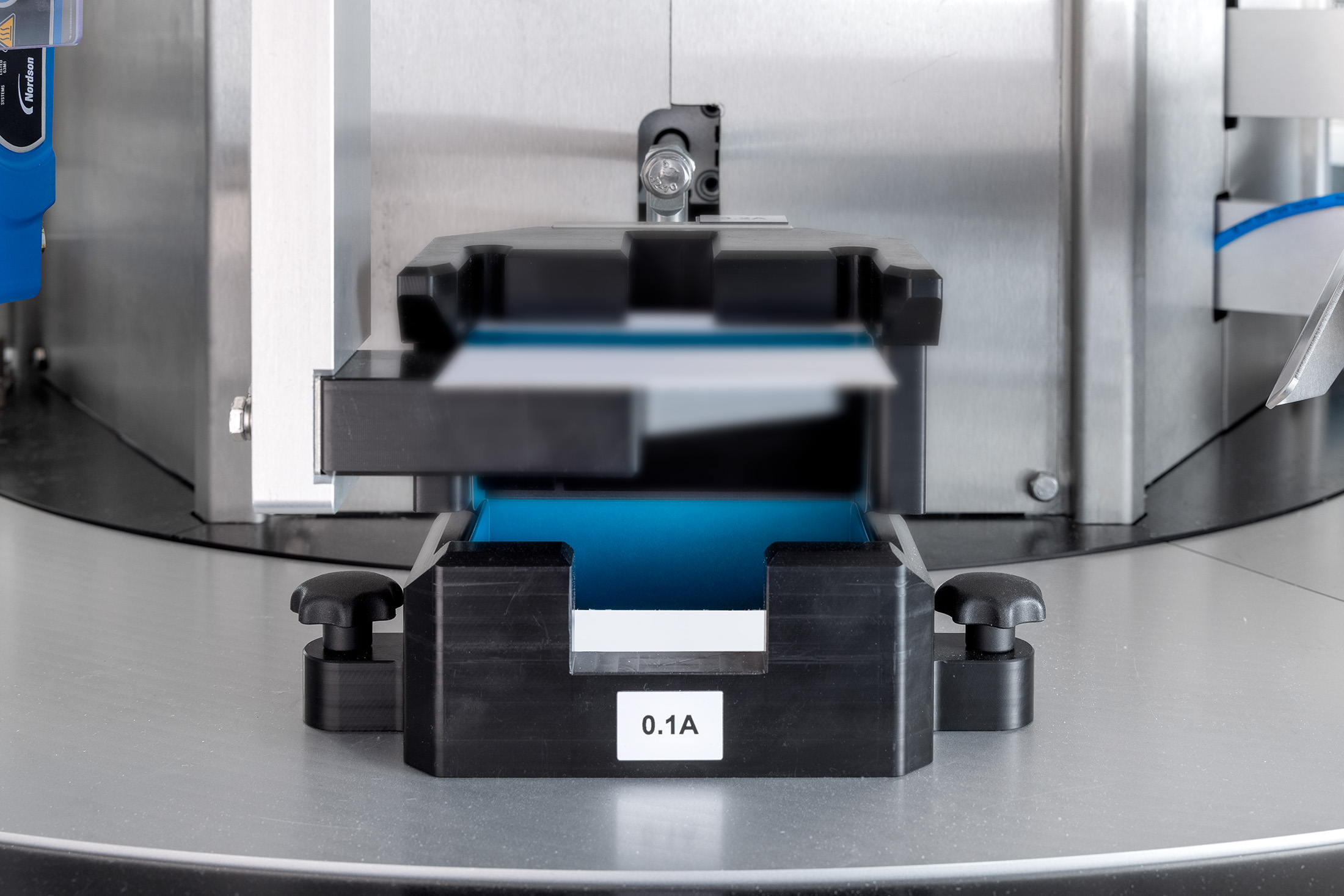
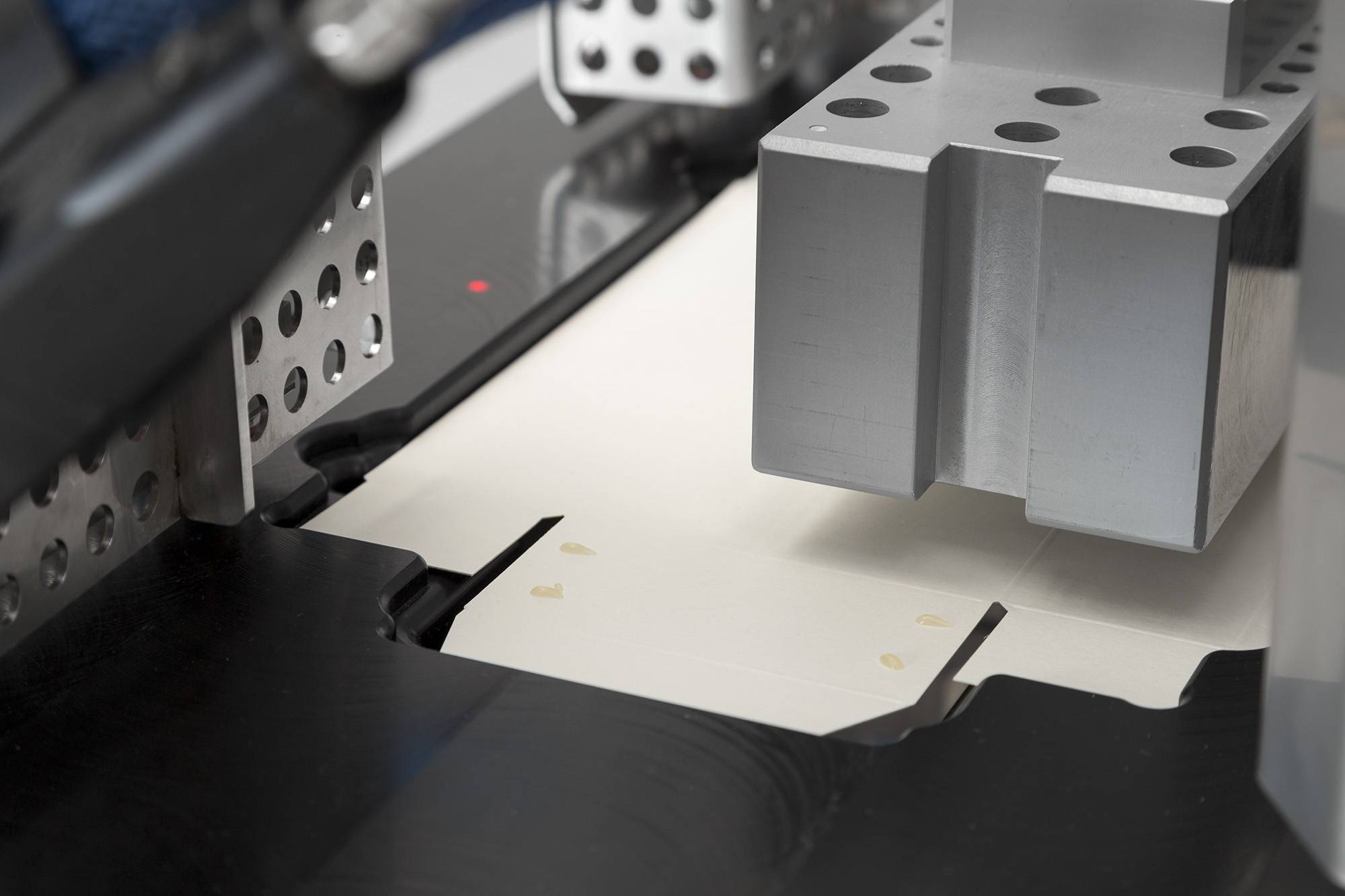
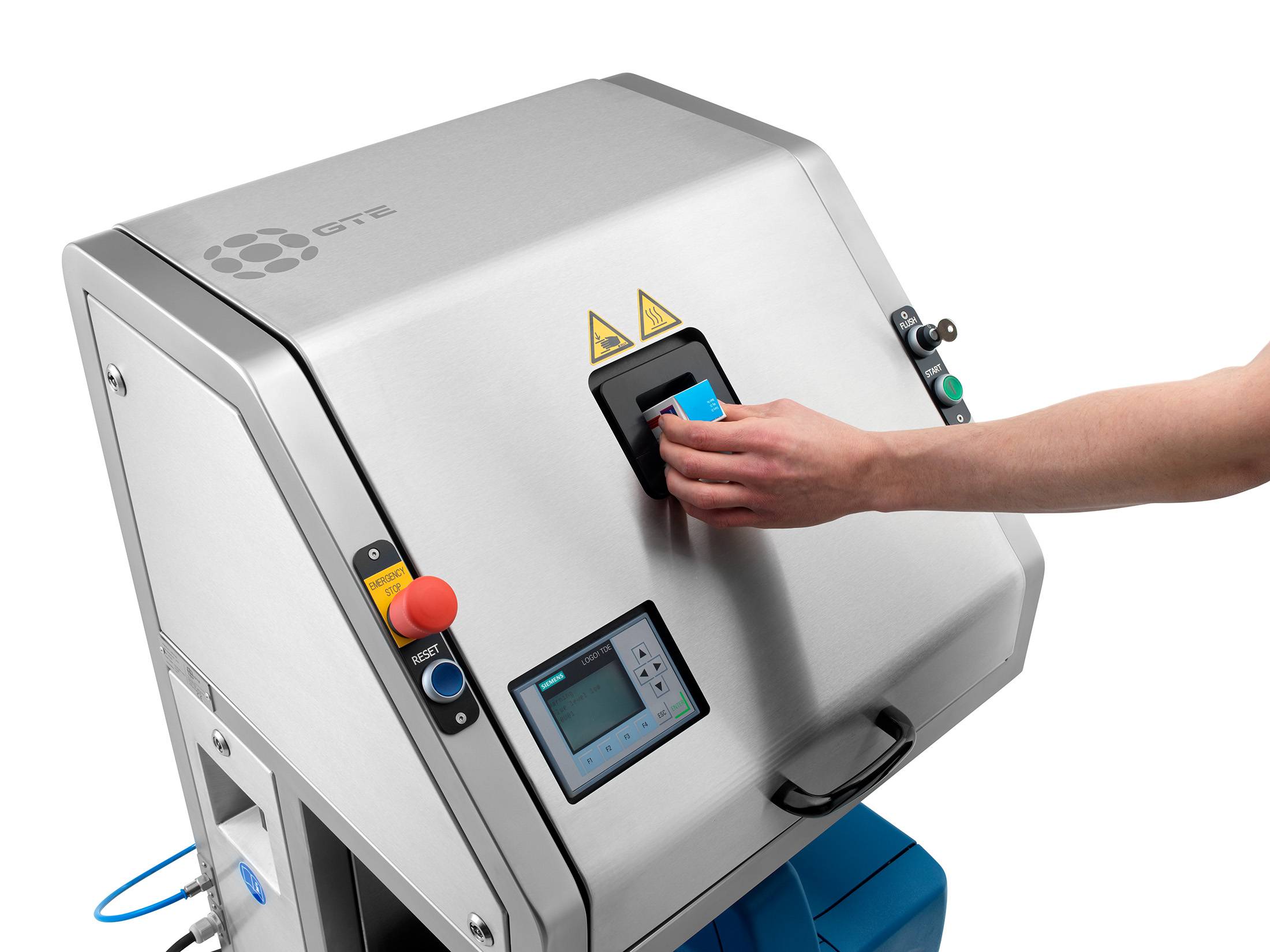
Logging process data
Logging process data is an important step within a production process. It allows you to keep control over what your machines do and makes it easy(er) to track errors and to find the cause of any recurring errors. In addition, if logging is combined with serialisation capability, batch data is also logged. This allows you to quickly find out where the production error occurred in a particular batch.

Serialisation
If you’re looking for an easy way to find out which products were made in the same batch, serialisation is the answer you’re seeking. Serialisation is the process of printing a batch code is on product packaging or the product itself. If you need to recall a batch of products, for example, you and your customers can easily check if you have products that should be returned.
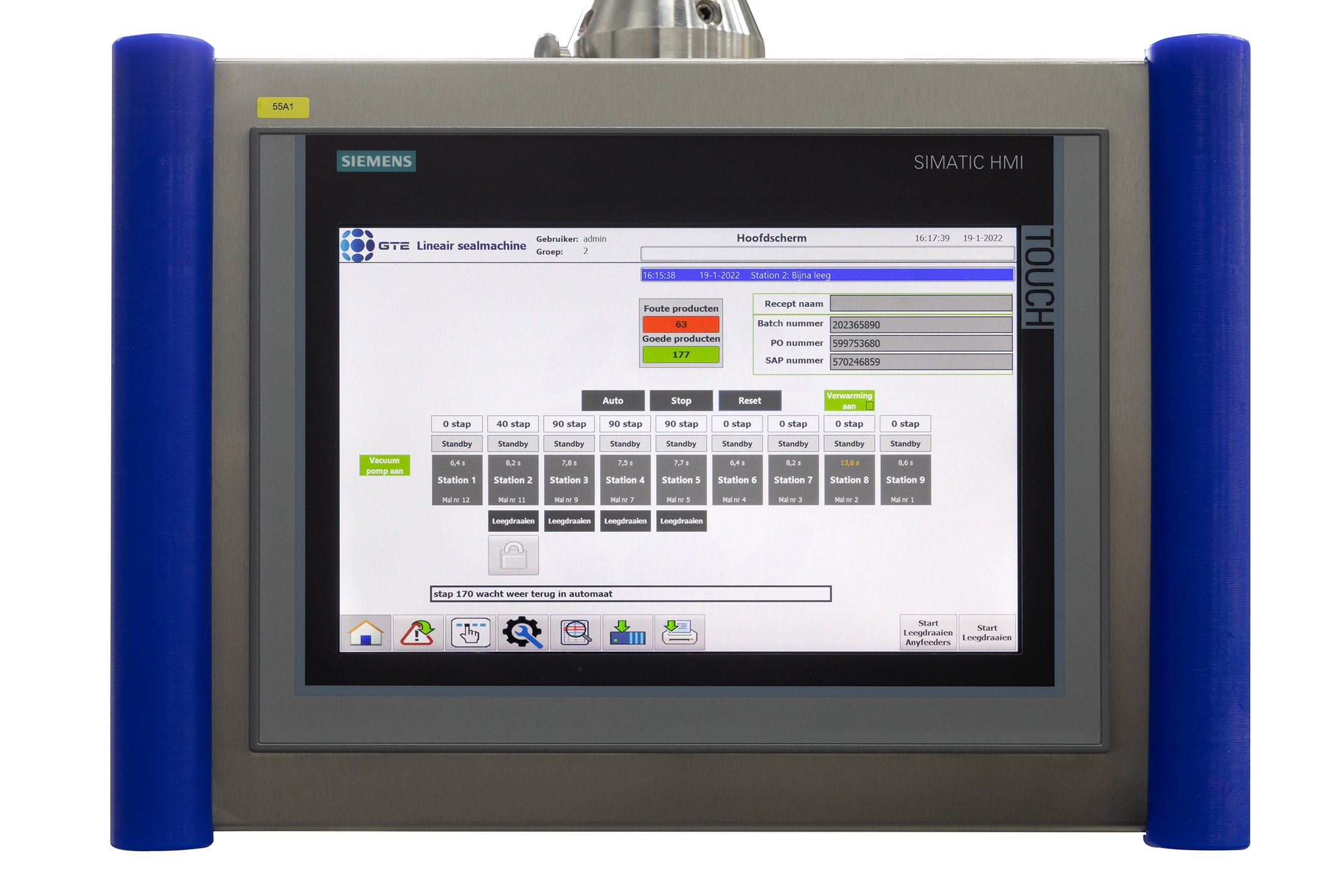
Complete control with logging & serialisation
Thanks to GTE Engineering’s many years of experience in the field of logging & serialisation, we’re more than able to advise you on the possibilities that Logging & Serialisation has to offer you. This includes discussing MES, SCADA, OEE, and ERP systems to see which one best suits your process. After implementing them, controlling and detecting errors within your production process will be a cakewalk.
Get more information
Wondering how the Machine could be useful for your organisation? Request more information using the form below.
"*" indicates required fields

Knowledge & Inspiration

Whitepaper: Everything about vision systems in the pharmaceutical and medical industry
Precision and accuracy are extremely important. Whether they are used for correctly labeling products, or verifying the integrity of packaging, vision systems play a crucial role in any production process. What are their strengths and limitations, how do you prepare for their implementation, and what will the future look like in terms of, for example, AI? We are happy to help you get started; download the free whitepaper!

The Importance of IQ, PQ, and OQ in Machine Construction for the Pharmaceutical and Medical Industry
In the pharmaceutical and medical industries, developing machines that meet the highest quality and regulatory standards is essential. Therefore, the steps of IQ (Installation Qualification), PQ (Performance Qualification), and OQ (Operational Qualification) are followed during the construction of these machines. These qualification tests ensure that machines meet the required standards for installation, operation, and performance, and they are a critical part of the validation process.
At GTE Engineering, we use a traceability matrix and provide support with FAT (Factory Acceptance Test) and SAT (Site Acceptance Test) protocols to make the validation process structured and efficient. This approach guarantees that all requirements are documented and that the machines meet the highest standards in the pharmaceutical and medical industries.
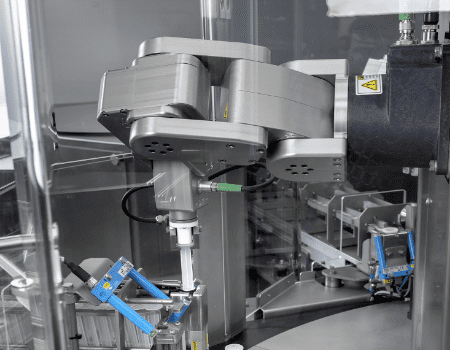
The LAP-C with Mecademic robot
GTE designed and built the LAP-C. A circular platform for assembling and/or packaging medical products. When we design the LAP-C as an assembly machine, we often use a Mecademic robot.

High mix low volume manufacturing
In the pharmaceutical industry, there is a growing demand for high mix low volume manufacturing (HMLV). HMLV signifies that medications and medical devices are increasingly being personalized, tailored more specifically to the patient: a greater variety of medical products combined in smaller quantities.

3D printing in engineering– thinking out of the box
Years ago, in 2001, GTE engineering embraced 3D printing for the creation process of its exclusive machines. Until then it was science fiction. Today we can no longer live without it. How do we use 3D printing at GTE and what advantages does it have?

What is ‘Smart Customization’?
ETO, CTO and Smart Customization. Terms that are more often used in mechanical engineering. But what do these terms mean? Everyone working in mechanical engineering should know what development these words describe.
Let us call you back
Curious about how our solutions can be used within your organization? Request more information quickly and easily.
"*" indicates required fields
We are ready to help you!
Mercuriusplein 45971 LW Grubbenvorst (Nederland)